

Facilitates real-time access to national guidelines for clinicians to ensure rational antimicrobial use at the point of care. With support from USAID’s Global Health Security Agenda and technical guidance from JBRSOFT, the STG App has been successfully piloted with 1000+ physicians across tertiary care hospitals in Bangladesh. The initiative is led by the Communicable Disease Control Programme under the Directorate General of Health Services, Ministry of Health and Family Welfare.
Click here to Read More

Empowering Cross-Sectoral AMR Surveillance and Action in Bangladesh

JBRSOFT has played a pivotal role in designing and developing the National One Health AMR Portal—an interoperable digital platform hosted by the One Health Hub of Bangladesh. This portal integrates and visualizes AMR, AMU, and AMC data across the human, animal, aquaculture, and environmental sectors, supporting informed policy decisions and multisectoral collaboration. As part of the national AMR containment effort, we successfully developed and deployed two dedicated platforms for the Communicable Disease Control (CDC), DGHS, supporting:
By streamlining data collection, analysis, and dissemination, JBRSOFT has empowered key stakeholders—DGHS, DLS, DoF, and DoE—with a unified digital infrastructure to combat AMR collaboratively under the One Health approach.
Are you interested in similar platform? Get in Touch with UsDigitized WHO’s Infection Prevention and Control Assessment Framework (iPCAMS) and Antimicrobial Stewardship (AMS) tools to streamline facility assessments and data reporting.


The iPCAMS is a web-based and mobile application that enables baseline assessments of infection prevention control and antimicrobial stewardship at hospital facilities to enhance infection prevention. It provides a national and hospital-level dashboard where you can monitor and compare the progress of the IPC and AMS.

iPCAMS findings assists the hospital infection prevention committees to develop time bound IPC and AMS action plans along with the person responsible for each action and initiate using continuous quality improvement (CQI) methods for achieving improvements to reduce healthcare associated infection (HAI).
Read More




Deployed PVIMS for pharmacovigilance monitoring to strengthen drug safety and regulatory workflows.
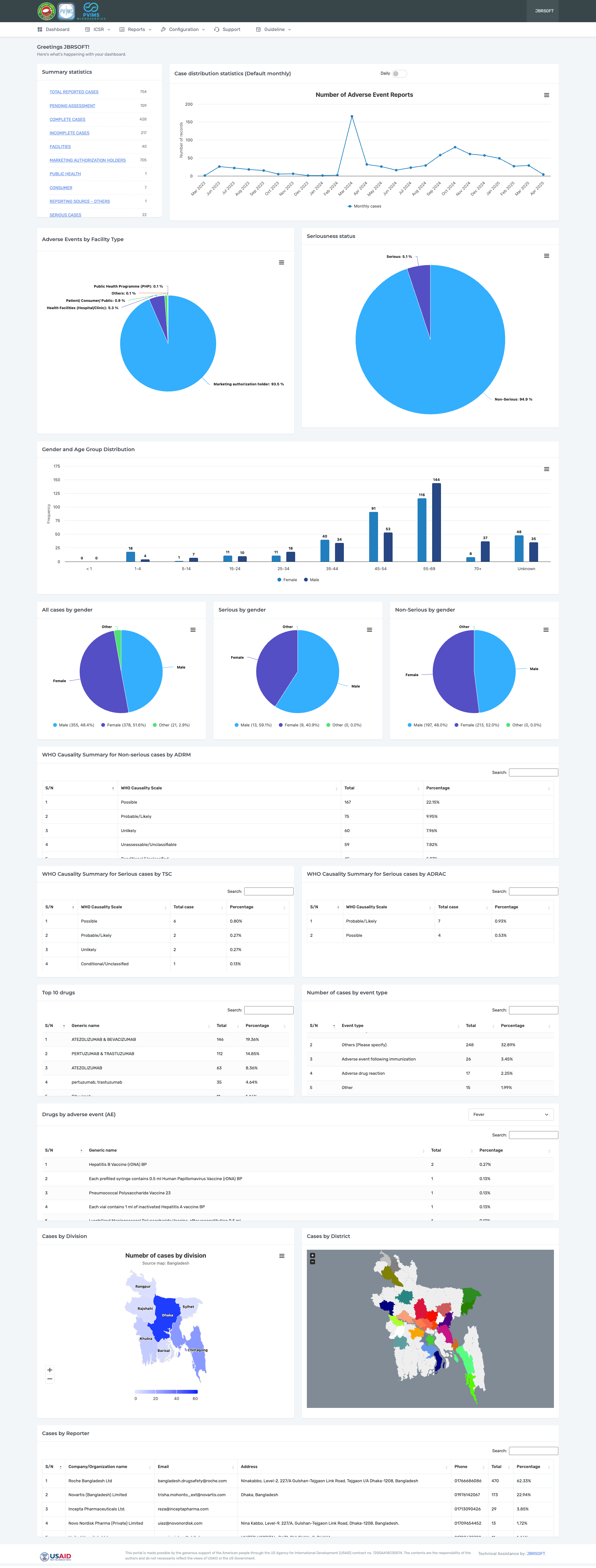
PViMS, or the PharmacoVigilance Monitoring System, is a web-based application used to monitor the safety of medicines. The application was developed by the USAID-funded Systems for Improved Access to Pharmaceuticals and Services [SIAPS] program (2011-2018) and is implemented by the USAID-funded Medicines, Technologies, and Pharmaceutical Services [MTaPS] program (2018-2023), both led by Management Sciences for Health (MSH), a global health nonprofit. PViMS is maintained by MSH.
JBRSOFT, a leading software development company in Bangladesh, has been working closely with the Directorate General of Drug Administration and the MSH team to customise the PViMS for the country context, build the Microservice, and provide technical support to users.
The PViMS is a comprehensive digital platform designed to strengthen medicine safety surveillance and reporting. At its core, PViMS empowers users to report adverse drug reactions (ADRs) through an intuitive YellowCard module, supported by a dedicated user panel that allows individuals to track, update, and manage their case submissions. The system also features an advanced admin panel that facilitates complete oversight, case management, and data-driven reporting, ensuring that health authorities can effectively monitor and respond to medicine-related safety events.
PViMS is built with scalability and global integration in mind. Its interoperability module enables seamless data sharing with the World Health Organization’s Uppsala Monitoring Centre (WHO UMC), adhering to international pharmacovigilance standards. Additional features include real-time live support, flexible system configuration options, and a comprehensive user guideline module, all of which contribute to building a user-centric, transparent, and efficient national pharmacovigilance ecosystem.





A robust inventory and prescription system supporting antibiotic stewardship, now enhanced with adherence tracking features (DoseGuard integration).
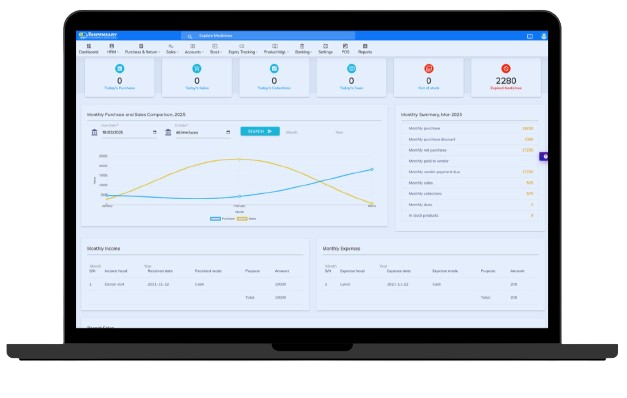
Dispensary is a comprehensive pharmacy management system designed to enhance drug management, patient counseling, and operational efficiency in pharmacies and hospitals. In many developing countries, challenges such as improper patient counseling, poor drug storage conditions, and inefficient pharmacy operations lead to medication wastage and delays in patient care. Our system digitalizes pharmacy operations, ensuring better stock control, faster service, and seamless integration with existing EMR, EHR, or hospital management systems through our web API. If you want to improve pharmacy service quality while reducing costs, Dispensary offers an affordable and efficient solution.
In June 2024, the U.S. Pharmacopeial Convention (USP), in collaboration with the Directorate General of Drug Administration (DGDA) and with support from the USAID-funded Promoting the Quality of Medicines Plus (PQM+) program, launched the DGDA Digitization Roadmap Development initiative in Bangladesh. This initiative commenced with the first Technical Working Group (TWG) meeting and marked a pivotal step toward modernizing the pharmaceutical regulatory framework in the country. The project focused on assessing DGDA’s existing IT infrastructure, identifying key gaps, and developing a comprehensive roadmap to enhance its regulatory processes. The overarching goal is to support DGDA in reaching WHO Maturity Level 3 and achieving WHO prequalification for the National Drug Control Laboratory (NDCL), thereby strengthening regulatory oversight, transparency, and alignment with global standards.
JBRSOFT successfully executed this critical assignment and formally handed over the digitization roadmap to the USP/PQM+ and DGDA teams during an official inauguration ceremony held at the DGDA auditorium on December 23, 2024.






The better way to survey and assess your health facility with Survey AMR
SurveyAMR stands at the forefront of the battle against antimicrobial resistance (AMR) for several compelling reasons:
In summary, SurveyAMR is uniquely positioned to address the complex challenges posed by AMR. Join us in our mission to combat antimicrobial resistance and build a healthier future for generations to come.